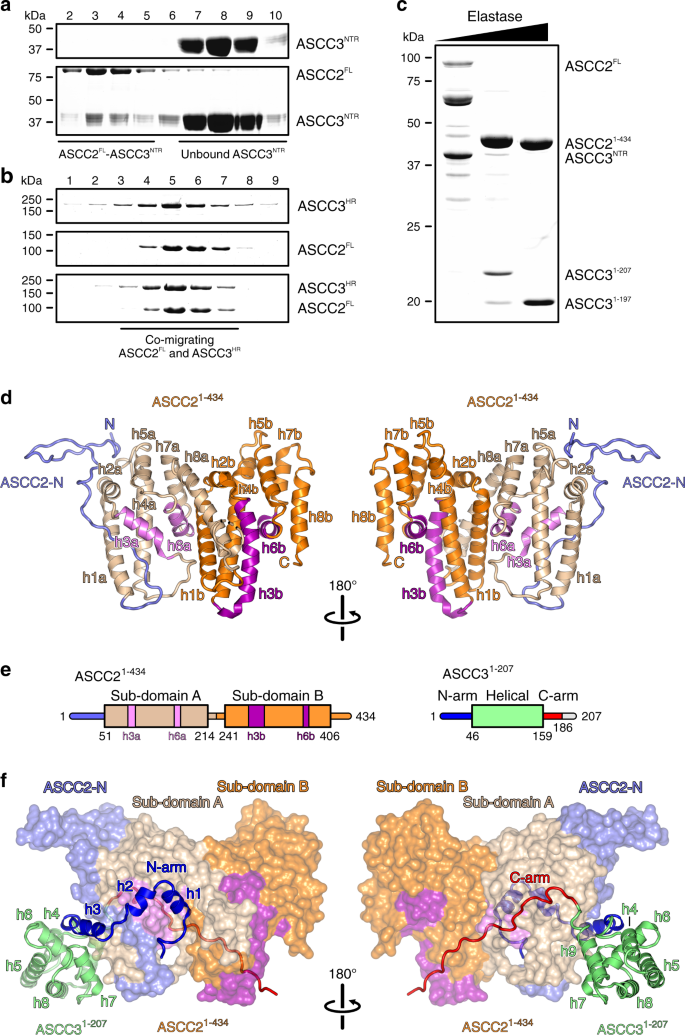
Experimental definition of a stable, minimal ASCC2–ASCC3 complex
Full-length ASCC2 (ASCC2FL) and two fragments of ASCC3, encompassing the NTR (ASCC3NTR; residues 1–400) and helicase region (ASCC3HR; residues 401-2202), were obtained by recombinant expression in insect cells. Analytical size exclusion chromatography (SEC) showed that ASCC2 interacts with ASCC3NTR (Fig. 1a) but not with ASCC3HR (Fig. 1b), consistent with previous reports9. The complex assembled from ASCC2FL and ASCC3NTR failed to crystallize. To remove putatively flexible regions that may hinder crystallization, we subjected the ASCC2FL–ASCC3NTR complex to limited proteolysis and mapped stable fragments by mass spectrometric fingerprinting. Elastase digestion gave rise to an approximately 50 kDa fragment of ASCC2, containing the first 434 residues (ASCC21–434), and an N-terminal, 207-residue fragment of ASCC3 (ASCC31–207), which maintained stable interaction in SEC (Fig. 1c). The ASCC21–434–ASCC31–207 complex was produced by recombinant co-expression in insect cells, purified and yielded diffracting crystals.
a, b SDS-PAGE analysis of SEC runs monitoring interaction of ASCC2FL and ASCC3NTR (a) and lack of interaction between ASCC2FL and ASCC3HR (b). In this and following figures: kDa, molecular weights of molecular weight markers in kDa. c SDS-PAGE analysis of elastase treatment of the ASCC2FL-ASCC3NTR complex. Bands running between ASCC2FL and ASCC21–434 represent intermediate ASCC2 fragments that occur transiently in the course of the digestion. Experiments shown in a–c have been repeated independently at least three times with similar results. d Orthogonal cartoon plots of ASCC21–434. ASCC2-N, N-terminal extension, slate blue; helices in sub-domain A, beige and violet; helices in sub-domain B, orange and purple. Helices are labeled as in the text. N, N-terminus; C, C-terminus. e Schemes of the domain organizations of ASCC21–434 and ASCC31–207. Violet and purple bars in the ASCC21–434 scheme represent helices h3a/b and h6a/b. The C-terminal 21 residues of ASCC31–207 (gray line) are not defined in the electron density. f Orthogonal views on the ASCC21–434-ASCC31–207 complex, with ASCC21–434 as surface view and ASCC31–207 as cartoon. ASCC21–434, colored as in d. N-arm of ASCC31–207, blue; C-arm of ASCC31–207 complex, red; helical domain of ASCC31–207, lime green.
Structure analysis and overall architecture
The crystal structure of the ASCC21–434-ASCC31–207 complex was determined via the single wavelength anomalous dispersion (SAD) approach, using a complex reconstituted by SEC from seleno-methionine (SeMet)-derivatized ASCC21–434 produced in E. coli and ASCC31–207 produced in insect cells (Supplementary Fig. 1). The structure was refined at 2.7 Å resolution to Rwork/Rfree values of 20.4/24.7% with good stereochemistry (Supplementary Table 1). An asymmetric unit of the crystals contains one ASCC21–434–ASCC31–207 complex. In the final model, we traced residues 2–408 of ASCC21–434 with a gap between residues 216–226 representing a flexible loop. The ASCC31–207 model comprises residues 1–186 without gaps and two additional residues at the N-terminus that were retained after tag cleavage.
Within the complex, the structure of ASCC21–434 exhibits an irregularly structured N-terminal region (residues 2–50), and two similarly structured, helical sub-domains (sub-domain A, residues 51–214; sub-domain B, residues 241–406; Fig. 1d, e). Both sub-domains are composed of eight α-helices (h1a-h8a/h1b-h8b; Fig. 1d). Within each sub-domain, h1/h2, h4/h5, and h7/h8 form helical hairpins (beige/orange in Fig. 1d) that are connected by h3 and h6 (pink/magenta in Fig. 1d). h3a is significantly shorter than h3b (13 vs. 26 residues, respectively) and connected to h4a by a 13-residue, irregularly structured loop, as opposed to the single-residue connection between h3b and h4b. Furthermore, helices h7a and h8a are pried open compared to the h7b-h8b equivalents, due to helix h1b that wedges in between them (Fig. 1d).
The ASCC31–207 fragment folds into a central helical domain (residues 46–159) and extended arms at the N-termini and C-termini (N-arm, residues 1–45, C-arm, residues 160–186), with which it clasps the compact ASCC21–434 (Fig. 1e, f). The N-arm of ASCC31–207 forms three helices, h1, h2 and h3, preceded and connected by irregularly structured linkers along one flank of ASCC21–434 (Fig. 1f). The first helix (h1, residues 5–13) is embedded between helices h6a and h8a of ASCC21–434 sub-domain A and helix h1b of ASCC21–434 sub-domain B. Helix h2 (residues 15–21) runs across ASCC21–434 helix h3a. Helix h3 (residues 26–40) secures part of the N-terminal extension of ASCC21–434 between helices h1a and h3a of ASCC21–434 sub-domain A (Fig. 1f). The central helical region of ASCC31–207 consists of six helices (h4-h9). Helices h4, h5, h7, h8, and h9 are arranged in a circle around the central helix h6, in front of helix h2a of ASCC21–434 (Fig. 1f). The C-arm stretches in an extended, irregular conformation along the opposite flank of ASCC21–434, connecting sub-domains A and B of ASCC21–434 (Fig. 1f).
Interfaces between ASCC3 and ASCC2 are evolutionarily conserved
Structurally or functionally important regions in proteins are often evolutionarily conserved. We analyzed conservation of the ASCC21–434–ASCC31–207 contact regions using the ConSurf server40. This analysis revealed that residues located at ASCC21–434–ASCC31–207 interface regions are highly conserved (Fig. 2). Residues 91, 93, 103, 163, and 251 of ASCC21–434 were assigned the highest conservation scores. These residues are located in helix h3a, h6a and h1b, and form a binding pocket for helix h1 of the ASCC31–207 N-arm (Fig. 2a, left). ASCC21–434 residues that form a binding surface for the ASCC31–207 C-arm are conserved to a lesser extent (Fig. 2a, right). Mirroring the conservation pattern on ASCC21–434, residues of the ASCC31–207 N-arm, in particular the first 16 residues, are significantly more conserved compared to other surface positions of the protein (Fig. 2b). This analysis suggests that interactions involving the N-arm of ASCC31–207 may be of particular importance for the formation of the ASCC21–434-ASCC31–207 complex, and that the ASCC21–434–ASCC31–207 interaction observed here is likely conserved in all organisms that contain these proteins.
a Residue conservation mapped to the surface of ASCC21–434 with ASCC31–207 shown as cartoon (colored as in Fig. 1f). b Residue conservation mapped on the structure of ASCC31–207 shown as cartoon, with ASCC21–434 in surface view (beige). Central legend, color code representing the degree of conservation of individual residues. Views as in Fig. 1d, f.
The N-terminal arm of ASCC3 is essential for stable binding to ASCC2
To elucidate the relative importance of different regions of ASCC31–207 for interaction with ASCC21–434, we used structure-informed mutagenesis in combination with analytical SEC and isothermal titration calorimetry (ITC). First, we designed sequential N-terminal truncations of ASCC31–207 with or without the C-arm. ASCC3 fragments lacking C-terminal residues not visible in the structure (ASCC31–197) or additionally lacking the C-arm (ASCC31–161) maintained stable interaction with ASCC21–434 in SEC (Fig. 3a). By contrast, deletion of the N-arm (ASCC342–197) abolished stable binding to ASCC21–434 in SEC (Fig. 3b), while a partial N-arm deletion variant (ASCC316–197) still co-migrated with ASCC21–434 in SEC (Fig. 3c).
a–c SDS-PAGE analysis monitoring SEC runs of the indicated mixtures of proteins. d–h ITC runs monitoring the interaction between the indicated pairs of proteins. Deduced Kd values are listed as means ± SD for runs for which affinities could be quantified. i Western blot monitoring pulldown of ASCC2 by the indicated ASCC3 constructs from cell extracts. j Quantification of the data shown in i. Columns represent means relative to ASCC3FL of n = 2 independent experiments, using the same biochemical samples. Open circles, individual measurements.
Quantifying binding affinities by ITC revealed a similar, yet more detailed, picture. ITC showed a Kd of 3.5 nM for the interaction of the complete ASCC3NTR and ASCC2FL (Fig. 3d). ASCC31–197 (lacking the C-terminal 10 residues of ASCC31–207 but containing all ASCC3 residues with well-defined electron density in the ASCC21–434-ASCC31–207 complex structure) and ASCC21–434 interacted with a similar affinity (Kd of 3.8 nM), suggesting that the fragments contained in our crystal structure encompass the entire ASCC2–ASCC3 interacting regions (Fig. 3e). Deletion of the entire C-arm of ASCC31–207 (ASCC31–161) led to an approximately 14-fold decreased affinity (Kd = 47.7 nM; Fig. 3f). Truncation of the N-terminal 15 residues that form helix h1 in the N-arm of ASCC31–197 reduced affinity to ASCC21–434 by more than two orders of magnitude (Kd = 483.0 nM; Fig. 3g). Lack of the entire N-arm of ASCC31–197 (ASCC342–197) completely abrogated the interaction, as no signal was detected in ITC measurements (Fig. 3h). Together, the above results indicate that the N-arm, and in particular the first 15 residues, of ASCC31–207 are essential for a stable binary interaction with ASCC21–434, consistent with its high degree of evolutionary conservation. The C-arm of ASCC31–207 contributes to the interaction with ASCC21–434 but it is not essential for the proteins to maintain a stable complex in SEC, consistent with a reduced but still high level of evolutionary conservation of the C-arm.
We next tested whether the interaction pattern observed using recombinant proteins in vitro also applies to the ASCC2–ASCC3 interaction in living cells. Expression constructs encoding C-terminally flag-tagged ASCC3FL and stepwise N-terminally truncated fragments (ASCC316-end, ASCC342-end, ASCC3207-end, and ASCC3401-end) were used to generate stably transfected HEK293 Flp-In cell lines for the tetracycline-inducible expression of these proteins. After induction, flag-tagged ASCC3 or fragments were captured on anti-flag beads and co-precipitation of ASCC2 was monitored by western blotting. In line with our in vitro interaction mapping, ASCC3 variants with increasing N-terminal deletions co-precipitated stepwise reduced amounts of ASCC2 (Fig. 3i). ASCC3 variants lacking 15, 41, 206, or 400 N-terminal residues, co-precipitated 73, 40, 26, or 16%, respectively, of the amount of ASCC2 associated with full-length ASCC3 (Fig. 3j). While based on our structural and mutagenesis data truncation of the N-terminal 206 or 400 residues of ASCC3 should abrogate direct ASCC2–ASCC3 interactions, ASCC3207-end and ASCC3401-end most likely still pulled down reduced amounts of ASCC2 (Fig. 3i, j), because other ASCC subunits also mediate indirect ASCC2–ASCC3 interactions in vivo.
ASCC2 and ASCC3 residue substitutions found in human cancers cluster at interfaces and lead to reduced affinities
223 and 652 somatic nonsense, missense or frame shift mutations in human cancer cell or tissue samples have been mapped to the ascc2 and ascc3 coding regions, respectively (https://cancer.sanger.ac.uk/cosmic). 123 and 95 of these mutations affect residues in ASCC21–434 and ASCC31–207, respectively. Strikingly, 16 of the missense mutations and three nonsense mutations affect residues E53, R58, T60, D65, L70, R96, Y97, D103, or R121 in ASCC21–434, and 17 or one, respectively, of these mutations affect residues R5, R11, S12, D28, R33, K165, or E181 in ASCC31–207, which all constitute direct contact points between the proteins in our crystal structure (Fig. 4a). Many of the remaining point mutations map to, and are expected to disturb, the globular portions of the proteins. In addition, there are three frameshift mutations that would affect almost the entire ASCC21–434 region or large parts thereof (E30, F124, G171 in ASCC2; Fig. 4a). 25 frameshift mutations map to residues F163 and G164 in ASCC3, affecting the entire C-arm of ASCC31–207 (Fig. 4a).
a Orthogonal views on the ASCC21–434-ASCC31–207 structure, highlighting interface residues and frameshift positions affected by somatic cancer mutations. ASCC21–434, beige; ASCC31–207, lime green; ASCC2 residues, brown sticks; ASCC3 residues, dark green sticks; first positions affected by frameshift mutations, red. Orientations of the panels as in Fig. 1d,f. b Details of the interaction networks involving cancer-related residues D103 of ASCC2, as well as R5 and R11 of ASCC3. Dashed lines, salt bridges. Rotation symbol, view relative to Fig. 1d,f, left. c–i ITC runs monitoring the interaction of ASCC21–434 with the indicated ASCC31–22 peptide variants. Deduced Kd values are listed as means ± SD for runs for which affinities could be quantified.
We thus surmised that many residue substitutions of ASCC21–434 or ASCC31–207 found in human cancers affect the ASCC2–ASCC3 affinity. To test this hypothesis, we conducted comparative ITC analyses using ASCC21–434 and peptides representing the N-terminal 22 residues of ASCC3 exhibiting wild-type (WT) sequence or residue substitutions R5G, R5C, R5H, R5L, R11C, or R11H. R5 residue substitutions are found in large intestine and lung adenocarcinomas, as well as in cervical and esophageal squamous cell carcinomas, while the R11 residue substitutions occur in endometrioid carcinoma and large intestine adenocarcinoma (https://cancer.sanger.ac.uk/cosmic). In our crystal structure, R5 of ASCC3 is positioned at the N-terminus of helix h1 and interacts with D103 of ASCC21–434 (D103 of ASCC21–434 is also affected by somatic cancer mutations), while R11 of ASCC3 lies at the C-terminal end of helix h1 and engages in hydrogen bonds and/or salt bridges with D63 and D92 in ASCC21–434 (Fig. 4b). We used ASCC3 peptides in these experiments as the high affinity observed in ITC between ASCC21–434 and ASCC31–197 or longer ASCC3 variants (Fig. 3d, e) may mask differences due to single residue substitutions.
WT ASCC31–22 bound to ASCC21–434 with a Kd of 2.0 µM (Fig. 4c). ASCC31–22 peptides bearing R5L or R5G substitutions weakened the interaction with ASCC21–434 approximately eight-fold and eleven-fold, respectively (Fig. 4d, e). ASCC31–22 peptides comprising R5H or R5C substitutions showed more than 20-fold reduced affinities compared to WT (Fig. 4f, g). R11H or R11C substitutions in ASCC31–22 completely eradicated binding to ASCC21–434 (Fig. 4h, i). These observations are consistent with the notion that reduced affinity to ASCC2 represents a means by which of R5 and R11 residue substitutions in ASCC3 contribute to cancer phenotypes.
The N-terminal cassette is an active helicase unit in ASCC3
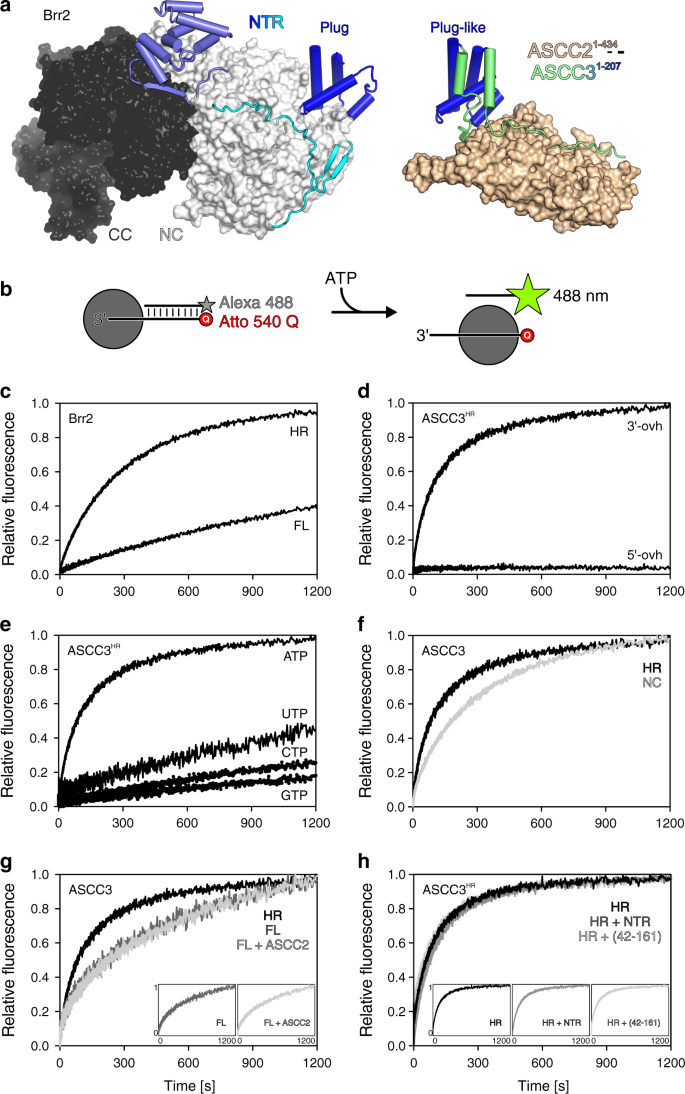
ASCC3 bears close resemblance to the spliceosomal RNA helicase, Brr2. Both ASCC3 and Brr2 contain an approximately 400-residue NTR followed by two Ski2-like helicase cassettes with identical domain composition (Fig. 5a, left). It is well documented that the N-terminal Ski2-like helicase cassette constitutes the active helicase unit in Brr2, while the C-terminal cassette lacks ATPase and helicase activities32. The reverse situation has been reported for human ASCC3; in contrast to an inactive isolated N-terminal cassette construct, an isolated C-terminal cassette construct was found to be active in DNA duplex unwinding8. As we did not perceive alterations in conserved helicase motifs in the N-terminal cassette that would obviously preclude helicase activity (Fig. 6a), we revisited the question of helicase activity of the ASCC3 helicase cassettes.
a Comparison of the structure of full-length yeast Brr2 (left) and the present ASCC21–434-ASCC31–207 complex (right) after superposition of the plug/plug-like domains (blue). Human Brr2 in the U4/U6•U5 tri-snRNP or in the pre-catalytic spliceosome exhibits a plug domain very similar to yeast Brr2 blocking RNA access42,43 (PDB IDs 3JCR, 6QW6, 6QX9). NC, N-terminal cassette; CC, C-terminal helicase cassette. b Experimental setup for stopped-flow/fluorescence-based unwinding assays. Gray sphere, helicase; star symbol, fluorophore (Alexa 488); Q, quencher (Atto 540 Q). c Stopped-flow/fluorescence-based assays monitoring unwinding of a 3’-overhang RNA by Brr2FL or Brr2HR, showing that auto-inhibition, which has been documented for Brr2 using gel-based unwinding assays33, can be readily detected using the present experimental setup. d Stopped-flow/fluorescence-based assays monitoring unwinding of DNA bearing a 3’-overhang (3’-ovh), and lack of unwinding of a 5’-overhang (5’-ovh) DNA by ASCC3HR upon addition of ATP. e Nucleotide preference of ASCC3HR in unwinding a 3’-overhang DNA. f Stopped-flow/fluorescence-based assays monitoring unwinding of 3’-overhang DNA by ASCC3NC compared to ASCC3HR using ATP. g Stopped-flow/fluorescence-based assays monitoring unwinding of a 3’-overhang DNA by the indicated ASCC3 constructs in the absence or presence of ASCC2 using ATP. Insets, side-by-side presentation of the largely overlapping curves. h Stopped-flow/fluorescence-based assays monitoring unwinding of a 3’-overhang DNA by ASCC3HR alone or in the presence of ASCC3NTR or ASCC342–161 using ATP. Insets, side-by-side presentation of the largely overlapping curves. FL/HR/NC/NTR/(42–161), ASCC3 variants as defined in the text.
a Multiple sequence alignment of conserved helicase motifs (indicated by letters or Roman numerals above the alignment) in human ASCC3, human Brr2 and yeast Slh1p N-terminal cassettes (NC) and C-terminal cassettes (CC). The conserved motif I lysine and motif II aspartate residues of ASCC3 cassettes, which were altered in the present analysis, are highlighted in blue and red, respectively. Color coding of the motifs: involved in ATP binding, blue; involved in RNA binding, yellow; involved in communication of RNA binding and ATPase activities, red. Roles of selected residues are indicated below the alignment. b–d Stopped-flow/fluorescence-based assays monitoring unwinding of 3’-overhang DNA by the indicated ASCC3HR variants using ATP. Schemes below the unwinding traces represent ASCC3HR with altered residues highlighted. WT/K505N/D611A/K1355N/D1453A/KK505/1355NN/DD611/1453AA, ASCC3HR variants as defined in the text.
While we were able to produce a recombinant ASCC3 fragment encompassing the N-terminal cassette (ASCC3NC; residues 401–1300), a fragment containing only the C-terminal unit could not be produced in soluble form. To test helicase activities, we pre-incubated ASCC3 variants (see below) with a 12-base pair DNA duplex containing a 31-nucleotide 3’-single-stranded overhang, and bearing a fluorophore (Alexa Fluor 488) on the 3’-terminus of the short strand and a quencher (Atto 540 Q) on the 5’-terminus of the long strand (Fig. 5b). As a control, we first tested the assay with Brr2 variants and an analogous RNA duplex. Fast mixing of Brr2FL or Brr2HR (residues 395–2129; a truncated version of Brr2 lacking most of the auto-inhibitory NTR, largely equivalent to ASCC3HR) pre-incubated with the RNA substrate and ATP in a stopped-flow device led to time-dependent increases in the Alexa Fluor 488 fluorescence, indicating RNA duplex unwinding (Fig. 5c). Unwinding occurred at a significantly higher rate with Brr2HR than with Brr2FL (Fig. 5c), showing that the stopped-flow/fluorescence setup can reliably monitor differences in helicase activity between alternative dual-cassette helicase constructs. As expected, ASCC3HR efficiently unwound a DNA duplex with 3’-single stranded overhang, but not a duplex with 5’-single stranded overhang (Fig. 5d), and preferentially used ATP for DNA unwinding (Fig. 5e). Surprisingly, however, in our hands, ASCC3NC also exhibited helicase activity, albeit reduced compared to ASCC3HR (Fig. 5f). These findings were confirmed using gel-based unwinding assays (Supplementary Fig. 2).
Nucleic acid helicases contain a number of conserved, functionally important sequence motifs in their RecA domain cores (Fig. 6a). In particular, a lysine in motif I is required for ATP binding, while an aspartate in motif II is crucial for coordinating a magnesium ion to trigger the hydrolysis of ATP (Fig. 6a), and substitutions of these residues are expected to strongly interfere with helicase activity. To further test the contributions of the N-terminal and C-terminal cassettes to the helicase activity of a dual-cassette ASCC3 construct, we individually substituted the corresponding lysine and aspartate residues in the N-terminal cassette (K505, D611), in the C-terminal cassette (K1355, D1453) or in both cassettes of ASCC3HR with alanine or asparagine, respectively. We verified the intended mutations by sequencing the final generation of baculoviruses used for production of the ASCC3HR variants (Supplementary Fig. 3). ASCC3HR variants that contained mutations in motifs I or II of the N-terminal cassette had strongly reduced or no detectable helicase activities (Fig. 6b). By contrast, ASCC3HR variants that contained residue substitutions K1355N or D1453A within the C-terminal cassette retained the activity of WT ASCC3HR (Fig. 6c). Again, gel-based unwinding assays using D611A and D1453A variants of ASCC3HR were consistent with these results (Supplementary Fig. 2). Also consistent with these findings, DNA helicase activities of the K505N/K1355N and D611A/D1453A double variants were almost fully abrogated (Fig. 6d). These results suggest that, contrary to previous observations8 and similar to Brr2 (ref. 32), the N-terminal cassette of ASCC3 is an active helicase in vitro. As the ASCC3NC helicase activity was reduced compared to that of ASCC3HR (Fig. 5f; Supplementary Fig. 2) it remains to be seen whether the C-terminal cassette is inactive but stimulates helicase activity of ASCC3NC, as has been observed for Brr2 (ref. 32), or whether ASCC3CC is an active helicase as well that contributes to the overall activity of ASCC3HR.
ASCC2 does not influence the helicase activity of ASCC3
The Brr2 NTR contains helical “plug” and PWI-like domains connected by extended, intrinsically disordered regions33,41. In isolated yeast Brr2 (ref. 33), as well as in human Brr2 in the U4/U6•U5 tri-snRNP or in the pre-catalytic spliceosome42,43, the NTR or part of it can fold back onto the helicase region, with the plug domain blocking substrate RNA loading (Fig. 5a, left), thereby auto-inhibiting Brr2 ATPase and helicase activities. Having established a similar functional organization of the helicase regions of ASCC3 and Brr2, we sought to investigate whether ASCC3 is also auto-regulated via its NTR.
While the overall sequence identity between human ASCC3 and human Brr2 is around 41%, the sequence identity is lower for their NTRs, around 20%. Despite the lower level of sequence conservation, structural comparison of ASCC31–207 with the NTR of yeast and human Brr2 revealed that a central, four-helix portion of ASCC31–207 (residue 59–142) closely resembles the Brr2 plug domain (root-mean-square deviation [rmsd] of 2.7 Å for 36 pairs of common Cα atoms; Fig. 5a). However, in our crystal structure, the ASCC31–207 arms neighboring the plug-like domain extend along ASCC21–434 (Fig. 5a, left); thus, when bound to ASCC2, these regions would not be available to fold back on the ASCC3 helicase region.
The helicase activity of ASCC3FL was significantly reduced compared to ASCC3HR, consistent with similar auto-inhibition as for Brr2 (Fig. 5g). When added in trans, ASCC3NTR or the plug domain-containing fragment, ASCC342–161, did not alter ASCC3HR helicase activity (Fig. 5h), indicating that the NTR has to be covalently connected to the helicase region to elicit the auto-inhibitory effect. Addition of ASCC2 did not influence the helicase activity of ASCC3FL (Fig. 5g). Due to the high affinities quantified for the ASCC2FL-ASCC3NTR and ASCC21–434-ASCC31–197 interactions (Fig. 3d, e), we consider it unlikely that the NTR is sequestered from ASCC2 by interacting in a mutually exclusive manner with the helicase region. Instead, the plug-like domain in ASCC3 may still be able to occupy an inhibitory position when associated with ASCC2.








 个人中心
个人中心 我的培训班
我的培训班 反馈
反馈












Comments
Something to say?
Log in or Sign up for free