Cancer immunotherapies have shown enormous promise, but they benefit relatively few patients. Overall response rates are only 15–20%. Even worse, whether a cancer immunotherapy will help an individual patient is usually unclear.
To develop more effective cancer immunotherapies and improve patient stratification, researchers are turning to various technologies that can detail the interactions between immune cells and cancer cells—interactions that may be attended by phenotypic changes. These technologies include high-content screening and live-cell imaging. Recently, these technologies have started moving from 2D cellular models to potentially more telling 3D cellular models and organoids.
 Several peer-reviewed studies have considered how 3D models could be useful in the development of new drugs and in studies of drug resistance. For example, researchers based at ETH Zurich reported on a novel three-dimensional heterotypic spheroid model for assessing the activity of cancer immunotherapy agents (Cancer Immunol. Immunother. 2017; 66(1): 129–140). The researchers asserted that their model provides “a novel and versatile tool for in vitro evaluation of cancer immunotherapy agents and allows for assessment of additional aspects of the activity of cancer immunotherapy agents, including analysis of immune cell infiltration and drug targeting.”
Several peer-reviewed studies have considered how 3D models could be useful in the development of new drugs and in studies of drug resistance. For example, researchers based at ETH Zurich reported on a novel three-dimensional heterotypic spheroid model for assessing the activity of cancer immunotherapy agents (Cancer Immunol. Immunother. 2017; 66(1): 129–140). The researchers asserted that their model provides “a novel and versatile tool for in vitro evaluation of cancer immunotherapy agents and allows for assessment of additional aspects of the activity of cancer immunotherapy agents, including analysis of immune cell infiltration and drug targeting.”
Also heralding the transition from 2D to 3D modeling are platform developers such as PerkinElmer. “There is a need to better understand the interactions between cancer and the immune system to guide new immunotherapy strategies, including combination therapies,” says Anis H. Khimani, PhD, senior strategy and market segment leader at the company. He notes that it is important to find biomarkers that will help researchers elucidate biology and improve patient stratification.
“PerkinElmer has taken a holistic approach to enable translational research to evaluate single biomarkers as well as perform multiparametric analysis within a single sample,” he continues. “High-content imaging and multiparametric studies, using the Opera Phenix® Plus High Content Screening system, along with the Columbus™ image data storage and analysis software, enable the deciphering of subcellular-content-rich information on immune biomarkers from both 2D and 3D cellular tumor models.”
The prospects for high-content screening in a 3D context were discussed in a paper (Comp. Med. Chem. III 2017: 388–415) by University of Colorado researchers. “The marriage of automated high-content sequencing with 3D models of human disease holds the potential to discover more effective drug therapies,” they wrote. “Moreover, organoids can be
produced from primary human tissues and genetically functionalized to study individual human disease or even populations, providing a platform to develop precision medicines.”
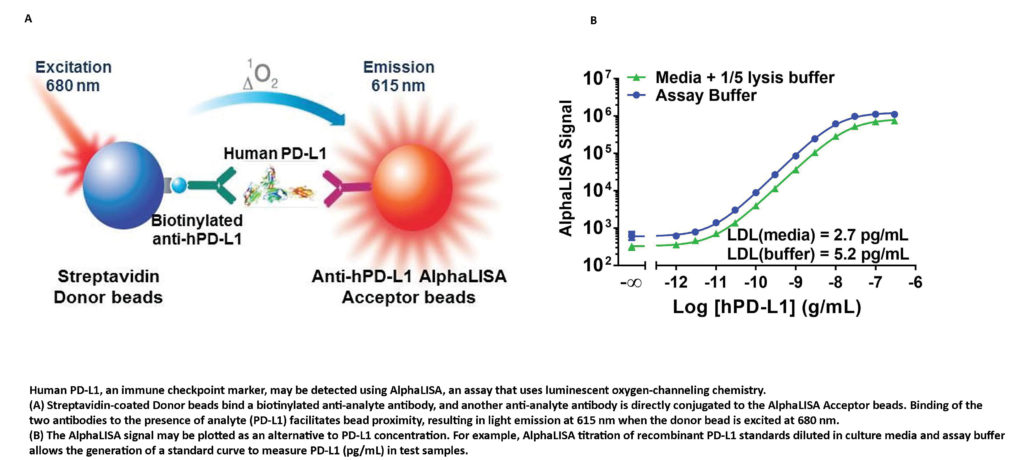
“In addition to cellular imaging applications in preclinical and clinical research,” Khimani says, “upstream drug discovery and preclinical development workflows have been enabled via the AlphaLISA®, DELFIA® TRF, and HTRF® multimode detection technologies for immuno-oncology research and screening.” For example, as described in an application note by Jeanine M. Hinterneder, PhD, senior applications scientist at PerkinElmer, the AlphaLISA® technology has been used to identify and characterize endogenous PD-L1 expression in breast cancer cells.








 个人中心
个人中心 我的培训班
我的培训班 反馈
反馈












Comments
Something to say?
Log in or Sign up for free