In a recent study published in the journal Emerging Infectious Diseases, scientists detected Histoplasma capsulatum deoxyribonucleic acid (DNA) in soil and penguin fecal samples in Antarctica, highlighting the harmful effects of increased human activity on the continent.
 Dispatch: Molecular Detection of Histoplasma capsulatum in Antarctica. Image Credit: SZakharov / Shutterstock
Dispatch: Molecular Detection of Histoplasma capsulatum in Antarctica. Image Credit: SZakharov / Shutterstock
Background
Histoplasmosis is an infection caused by inhaling Histoplasma capsulatum spores found in bird and bat droppings and dispersed due to environmental and anthropogenic disturbances.
Histoplasma capsulatum belongs to the order Onygenales and is known to cause systemic mycosis in many regions of Africa, North America, South Asia, and central and south America, with the incidence highest in Latin America. Recent whole-genome studies have proposed four genetically different Histoplasma species — H. capsulatum (Panamanian lineage), H. mississippiense (North American lineage), H. ohiense (North American lineage), and H. suramericanum (Latin American lineage).
Despite its inhospitable nature, the last two decades have seen increased human activity in Antarctica in the form of fishing, whaling, scientific research, and tourism. The influx of humans has brought non-indigenous species such as pathogenic fungi to the continent. In addition, it has resulted in the dispersal of Antarctica’s autochthonous and endemic species to other landmasses. Given the evidence on how foreign flora and fauna can disrupt native ecosystems, it is imperative to monitor the introduction of non-endemic species into a landmass.
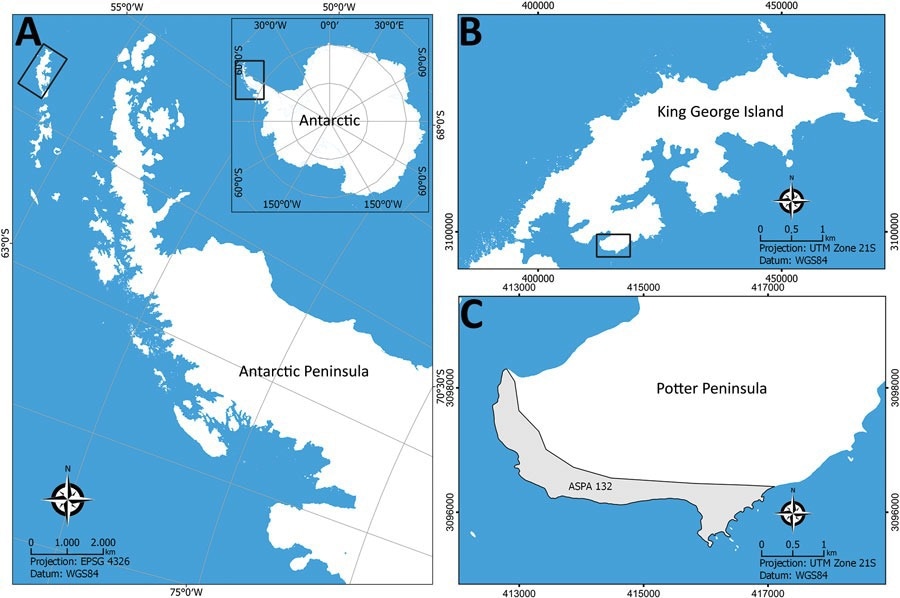
Sampling locations for study of Histoplasma capsulatum in Antarctica. A) Location of the Antarctic Peninsula in the Antarctica continent; B) King George Island; C) Potter Island and the Antarctic Specially Protected Area ASPA N°132. Source: SCAR Antarctic Digital Database (https://www.scar.org/resources/antarctic-digital-database).
About the study
In the present study, the researchers collected soil samples and penguin and fur seal fecal samples from an Antarctic Special Protected Area on King George Island known as Potter Peninsula.
Genomic DNA extraction and nested polymerase chain reaction (PCR) were carried out to generate sequences of a 100-kilodalton (kDa)-like protein gene. These sequences were compared with other H. capsulatum sequences from the GenBank sequence database. The sequences were then used to build a phylogenetic tree to infer the evolutionary history of H. capsulatum from Antarctica.
Results
The results reported the presence of H. capsulatum from two of the eight soil samples and three of the nine penguin fecal samples collected. The phylogenetic tree revealed different affinities for the five sequences generated in this study. The genetic data from one soil and two fecal samples grouped with the Latin American LAmB1 lineage, while the remaining soil and fecal sample sequences clustered with sequences from the other Latin American lineage LAmA2. None of the sequences generated in this study showed affinities to the North American or Panamanian lineages.
Potter Peninsula, being a protected area, is home to many migratory birds during the summer months and plays host to bird and breeding marine mammal colonies. All the faunal activity has resulted in soil rich in nitrogen, calcium, phosphorous, and organic carbon.
Histoplasma capsulatum generally requires a temperature range of 18–28°C and low-lit environments with humidity above 60% to thrive. The authors believe that the H. capsulatum species complex succeeds in surviving in the less-than-ideal conditions of Antarctica because of the high organic content of the soil in the Antarctic Special Protected Area, which promotes fungal growth. Furthermore, they hypothesize that the fungus could have dispersed onto the landmass with the migratory birds or humans coming to Antarctica.
Given the different phylogenetic affinities of the H. capsulatum sequences from Antarctica, the researchers believe that there could have been multiple dispersals of the fungus or there have been adaptive changes in the species. The phylogenetic results also indicated the shared geological history of Antarctica and South America.
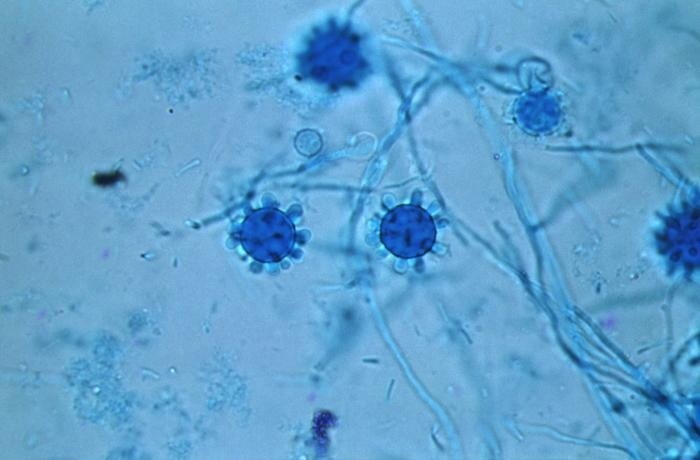
Under a magnification of 800X, this photomicrograph revealed some of the ultrastructural details exhibited by Histoplasma capsulatum fungal organisms, which had been extracted from an isolate that included a number of tuberculate, spheroidal macroconidia, and diaphanous filamentous hyphae.
Conclusions
To conclude, the study detected H. capsulatum DNA in soil and penguin fecal samples from a part of the Antarctic Special Protected Area, which is home to many migratory birds and marine mammal colonies. This finding suggests a much broader occurrence of the fungus than was previously suspected and also indicates the ability of H. capsulatum to survive at temperatures lower than its ideal growing conditions. The DNA sequences from the soil and fecal samples show different phylogenetic affinities, suggesting multiple dispersals.
According to the authors, given the ability of fungal pathogens such as H. capsulatum to cause widespread infections, surveillance of emergent pathogenic fungi and their transmission in Antarctica is fundamental in preventing histoplasmosis and other mycoses.








 User Center
User Center My Training Class
My Training Class Feedback
Feedback













Comments
Something to say?
Log in or Sign up for free