Characterisation of iridium(III) complexes
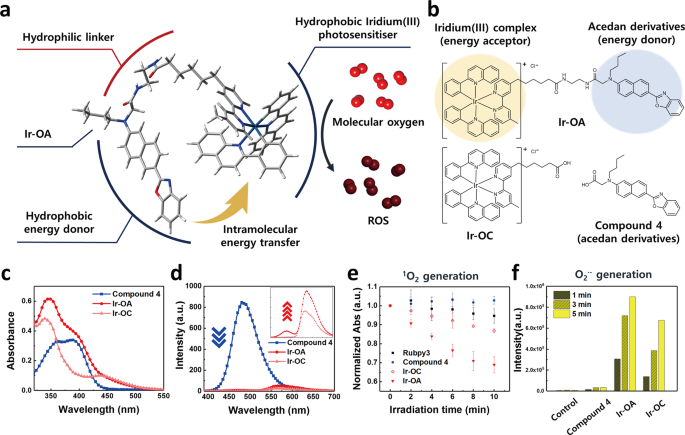
We synthesised iridium(III) complexes with (Ir-OA) and without (Ir-OC) energy donors (Fig. 1a, b, and Supplementary Figs. 1–9). Previous studies have revealed that cationic iridium(III) complexes with 2-phenylquinoline (2pq) ligands effectively generated a singlet oxygen (1O2) and superoxide radical anion (O2•–) owing to their suitable HOMO and LUMO levels for energy and electron transfers to molecular oxygen9. Nevertheless, the iridium(III) complex with 2pq ligands displayed an insufficient absorption coefficient (<6000 M−1cm−1) in the 400–500 nm range, resulting in a limited ROS production (Supplementary Table 1). Accordingly, we introduced an acedan (6-acetyl-2-(dimethylamino)naphthalene)-based energy donor to the iridium(III) photosensitiser (Ir-OA). Acedan derivatives have a strong absorption coefficient at 400 nm (>16,000 M−1cm−1) and high emission quantum yields (>0.90) in the range of 400–500 nm (Supplementary Table 1)20. The emission spectrum of energy donors and the absorption spectrum of the iridium(III) complex with 2pq ligands overlapped well (Supplementary Fig. 10), allowing efficient intramolecular energy transfer (Fig. 1a). The energy donor and acceptor of Ir-OA absorbed light independently due to its non-conjugated bridge, resulting in the absorption spectrum of Ir-OA corresponding to the sum of the absorption spectra of Ir-OC and compound 4 (Fig. 1c). In addition, the fluorescence of the energy donor at 470 nm was nearly quenched in Ir-OA (λex = 385 nm, the absorption peak of the energy donor). Simultaneously, the emission of the energy acceptor at 555 nm was enhanced, which strongly suggests that highly efficient intramolecular energy transfer occurred (φenergy transfer = 98.4%) (Fig. 1d).
a Schematic illustration of the molecular engineering strategy: intramolecular energy transfer and resulting photoactivated ROS generation. The illustrated Ir-OA molecule is a Δ isomer, but the Λ form enantiomer can exist. b Molecular structure of Ir-OA, Ir-OC, and compound 4. c UV-vis absorption spectrum of the three presented chemicals. d Subsequent emission spectra (λex = 400 nm) of the three chemicals. Magnified emission spectra show the enhanced emission of Ir-OA compared with that of Ir-OC, which reveals the evidence of energy transfer. Conditions for the absorption and emission spectra; [Ir-OA or Ir-OC or compound 4] = 20 μM in H2O:DMSO = 99:1 (v/v%). e 1O2 assay using the absorbance decay of 9,10-anthracenediyl-bis(methylene)dimalonic acid (ABDA) under light exposure. The ABDA is degraded by 1O2 produced by photoactivation of the Ir-OA. Data are presented as mean value ± s. d. (n = 3). f O2•– assay using the fluorescence enhancement of dihydrorhodamine 123 (DHR123). DHR123 is oxidised to rhodamine123 by the produced O2•–, which enhances fluorescence signal. Conditions for ROS assays: [iridium(III) complexes] = 4 μM; [ABDA] = 100 μM or [DHR123] = 4 μM in H2O:DMSO = 999:1 (v/v%). Source data are provided as a Source Data file.
In vitro/live cell ROS generation
To verify the capacity of Ir-OA and Ir-OC to generate ROS, we conducted an 1O2 generation assay using 9,10-anthracenediyl-bis(methylene) dimalonic acid (ABDA), and an O2•– assay using dihydrorhodamine123 (DHR123)21. Ir-OA in the ABDA solution showed greater 1O2 generation compared with Ir-OC, compound 4, and [Ru(bpy)3]2+ as a reference photosensitiser (Fig. 1e). Moreover, Ir-OA showed much stronger O2•– generation than Ir-OC and compound 4 in the DHR123 assay (Fig. 1f)22. This implies that more triplet excitons were produced by intramolecular energy transfer of Ir-OA, which accelerated 1O2 and O2•– generation. The ROS indicator 2′,7′-Dichlorodihydrofluorescein diacetate (H2DCF-DA) was utilised to confirm whether ROS were generated inside live cells by the photoactivation of Ir-OA (Supplementary Fig. 11)23. When HeLa cells were treated with Ir-OA and H2DCF-DA, photoactivation of Ir-OA under very weak light irradiation (LED array, λ = 400 nm, 0.17 J cm−2) produced ROS efficiently, resulting in a strong green signal. However, Ir-OC did not effectively induce an ROS signal under the same conditions as the light energy was not sufficient for Ir-OC to generate adequate ROS levels. According to the intracellular ROS assay (Supplementary Fig. 11), Ir-OA can be employed as an effective photosensitiser to induce strong oxidative stress to live cells and is expected to induce physiological dysfunction resulting in cell death.
Localisation of iridium(III) complexes
Ir-OA and Ir-OC contain cationic iridium cores and lipophilic ligands, which target mitochondria due to the negative mitochondrial membrane potential (MMP) at the inner mitochondrial membrane (IMM)24. We determined the subcellular location of Ir-OA and Ir-OC by confocal laser scanning microscopy (CLSM) using MitoTracker Deep Red, a fluorescent probe for IMM and intermembrane space (IMS) staining25. Noticeably, the CLSM images overlapped with those of the MitoTracker (Pearson’s coefficient = 0.86 for Ir-OA and 0.74 for Ir-OC), which clearly indicated that Ir-OA and Ir-OC were located on the mitochondria (Fig. 2a and Supplementary Fig. 12). To identify the detailed location of Ir-OA in the mitochondrial ultrastructure, we conducted Airyscan confocal imaging (~120 nm)26. The Airyscan image of Ir-OA also overlapped with that of the MitoTracker and showed a clear boundary of the mitochondria corresponding to the IMM; this image also showed localisation of Ir-OA in the outer part of the IMM (Fig. 2b). To further distinguish the sub-mitochondrial localisation of Ir-OA, mitochondrial matrix targeted Mito-EGFP was transfected for matrix imaging with Ir-OA (Fig. 2c). The Airyscan image showed that Ir-OA was mainly located at the outer boundary of the Mito-EGFP signals, implying that the major population of luminescent Ir-OA is not in the mitochondrial matrix. This super-resolution imaging result suggests that the sub-mitochondrial localisation of Ir-OA might be from the IMM spreading to the IMS and outer mitochondrial membrane (OMM). Therefore, Ir-OA is expected to exert oxidative stress not only on mitochondrial proteins but also on proteins of subcellular organelles, such as the endoplasmic reticulum (ER)27 and peroxisomes28,29, in contact with the mitochondria, which may cause protein dysfunction, microenvironment changes, and cell death.
a Confocal images of Ir-OA with MitoTracker. Phosphorescence of Ir-OA (red) and fluorescence of MitoTracker (green) and merged image (λex = 405 nm for Ir-OA, λex = 647 nm for MitoTracker® Deep Red FM). Pearson’s coefficient was calculated using Image J software (R2 = 0.86). b Airyscan confocal image of mitochondria with Ir-OA (red) and MitoTracker (green, outer/inner mitochondria membrane) for identifying the specific location of Ir-OA. c Airyscan confocal image of mitochondria with Ir-OA (red) and Mito-EGFP (green, mitochondrial matrix). Line profiling for clarifying the specific location of each emission signal was followed by corresponding images (right). The Ir-OA signal is well merged with the MitoTracker signal on the outer/inner mitochondrial membrane and encloses Mito-EGFP signal of the mitochondrial matrix. The mitochondria of Airyscan images were pre-swelled by photoactivation (λex = 405 nm, 0.0125 mW) of the Ir-OA for identifying mitochondrial substructure (the mitochondrial swelling effect of the Ir-OA is explained later). Line profiling analysis was proceeded with Carl Zeiss ZEN 3.0 Blue software. Conditions: [Ir-OA] = 4 μM, [MitoTracker® Deep Red] = 100 nM, incubation time = 2 h and 0.5 h, respectively. All imaging was repeated three times independently, and each experiment showed similar results. Source data are provided as a Source Data file.
Mitochondrial oxidation-induced cell death
To confirm the resulting cell death by photoactivation of Ir-OA in mitochondria, we conducted the CCK-8 and MTT assays for quantitative cytotoxicity analyses and the live/dead assay with propidium iodide (PI) (dead cell indicator) and Calcein AM (live cell indicator)30. Cell viability dramatically decreased following photoactivation of Ir-OA under low light energy (0.08–0.25 J cm−2) (Fig. 3a, Supplementary Fig. 13, and Supplementary Table 2). However, Ir-OC was unable to cause significant changes in cell viability as the generated ROS levels were insufficient to trigger cell death under the same conditions. Notably, the IC50 value of Ir-OA obtained using the CCK-8 assay was considerably higher than that obtained using the MTT assay (Supplementary Table 2). This is because the formed formazan in the MTT assay depends on the mitochondrial dehydrogenase, while CCK-8 is activated by dehydrogenases from whole cells. Thus, the CCK-8 assay is more appropriate and reliable in measuring the phototoxicity of photosensitisers targeting the mitochondria. Furthermore, the phototoxicity results of Ir-OA from the CCK-8 and MTT assays corresponded to results obtained from the live/dead assay. Intense PI fluorescence for dead cells was shown in HeLa cells with Ir-OA, whereas HeLa cells with Ir-OC exhibited the green fluorescence of Calcein AM for live cells (Fig. 3b). In addition, cells with Ir-OA showed an enhanced PI signal within 90 min of light irradiation (LED array, λ = 400 nm, 0.17 J cm−2), implying that cell death was initiated in less than 2 h (Supplementary Fig. 14), possibly due to the strong oxidation of mitochondria. The live/dead assay was confirmed by fluorescence activated cell sorting (FACS) and assessed using 2D histograms (Fig. 3c and Supplementary Fig. 15). When Ir-OA was photoactivated, the number of HeLa cells with positive PI and negative Calcein AM dramatically increased, confirming that most of the cells were dead. In addition, the number of cells with positive Annexin V and negative PI (Q2) increased upon photoactivation with Ir-OA prior to the disruption of the cell membrane (positive PI) (Fig. 3d and Supplementary Fig. 15), showing that the dying cells undergo an early apoptosis stage. Hence, the photoactivation of Ir-OA triggered apoptotic cell death while that of Ir-OC did not alter cell viability.
a CCK-8 assay for quantifying the cytotoxicity of Ir-OA and Ir-OC with or without light irradiation for HeLa cells. Data are presented as mean value ± s. d. (n = 4). Conditions: photosensitiser iridium(III) complexes incubation time = 2 h, light source = 400 nm light LED array, light dose: 0.085, 0.170, and 0.255 J cm−2. b Live/dead assay for verifying phototoxicity of Ir-OA and Ir-OC. At 6 and 20 h after light irradiation, dead and live cells were stained using propidium iodide (PI, red) and Calcein AM (green), respectively (scale bars = 200 μm). Conditions: [Iridium(III) complex] = 8 μM, light source = 400 nm light LED array, light dose = 0.255 J cm−2. The experiment was repeated three times independently, and each experiment showed similar results. c Representative Calcein AM vs. PI flow cytometry plot for HeLa cells incubated with iridium complexes. d Representative Annexin V vs PI flow cytometry plot for HeLa cells incubated with iridium complexes. Q1 (right top): late apoptotic/necrotic cells, Q2 (right bottom): early apoptotic cells, Q3 (left bottom): viable cells, and Q4 (left top): necrotic cells. Conditions for flow cytometry: [Ir complex] = 8 μM, light source = 400 nm light LED array, light dose = 0.25 J cm−2. Source data are provided as a Source Data file.
Mitochondrial viscosity change by protein-crosslinking
ROS and following oxidative stress triggers a change in microenvironment such as viscosity16,31 and polarity15,32 at the specific region affected by the stress. Particularly, increased viscosity affects biomolecular interactions and metabolite diffusion, which hinders mitochondrial respiration and metabolism31,33 and, subsequently, induces cell death. Although protein crosslinking has been proposed as a possible cause of viscosity change, no experimental evidence has been previously reported.
Generally, ROS are known to crosslink proteins and generate protein aggregates9,34, which can increase the viscosity of the microenvironment. The viscosity change can affect the lifetime of iridium(III) complex; lifetime change was measured by time correlated single photon counting (TCSPC). Firstly, the viscosity sensitivity of the Ir-OA phosphorescence lifetime was measured in MeOH and glycerol. The phosphorescence lifetime of Ir-OA increased from 278 ns to 2293 ns as the glycerol content (v/v) increased from 0% (0.55 cP) to 95% (950 cP) (Fig. 4a and Supplementary Fig. 16). In addition, we confirmed that the increased protein concentration at the local area resulted in the phosphorescence lifetime enhancement of Ir-OA. Bovine serum albumin (BSA), known to increase the viscosity of solutions according to its concentration35, was dissolved in aqueous solutions of Ir-OA at the following concentrations: 0.0156, 0.0625, 0.250, 1.00, and 4.00 mg/mL (Supplementary Fig. 17). Further, the phosphorescence lifetime of Ir-OA was measured and showed a concentration-dependent increase from 664 ± 31 ns to 1912 ± 40 ns. This result implies that the local accumulation of proteins by photocrosslinking, and the corresponding viscosity increase, can be monitored by the change in the phosphorescence lifetime of Ir-OA. Accordingly, in HeLa cells, we used phosphorescence lifetime imaging microscopy (PLIM) to monitor changes in mitochondrial viscosity as a product of oxidative stress caused by Ir-OA (Fig. 4b). HeLa cells with Ir-OA exhibited an average lifetime of 866 ± 20 ns before light irradiation (LED array, λ = 400 nm, 0.17 J cm−2), which increased to 915 ± 14 ns following irradiation, likely due to the accumulation of crosslinked proteins. Then, we further investigated whether Ir-OA successfully induced protein crosslinking with light irradiation (LED array, λ = 400 nm, 1.28 J cm−2) in live cells. In addition, the photocrosslinking reaction by Ir-OA was confirmed in each organelle. First, we transfected four different EGFP constructs (Mito-EGFP, mitochondrial matrix; Sec61b-EGFP, ER membrane; PEX16-EGFP, peroxisome; and PTBP1-EGFP, nucleus) (Supplementary Table 3) in the cells, followed by incubation of Ir-OA in the presence or absence of light. Western blot signals of covalently crosslinked EGFP were observed for Mito-EGFP, Sec61b-EGFP, and PEX16-EGFP in the presence of light (hv+) (Fig. 4c–f, left and Supplementary Fig. 18). However, the signal on a gel with anti-EGFP did not change after photoactivation of Ir-OA in the nucleus (PTBP1-EGFP) as the nucleus is further from the mitochondria and has no direct contact site.
a Viscosity-dependent change in the lifetime of Ir-OA. The in vitro viscosity was precisely controlled with glycerol content change in MeOH (v/v, %) from 0% to 95%. Data are presented as mean value ± s.d. (n = 3). b Phosphorescence lifetime image (PLIM) of Ir-OA in mitochondria before and after photoirradiation. The described lifetime is averaged value of three images obtained under same condition (n = 3). c–f Western blot (left) for identification of protein photo-crosslinking of Ir-OA depending on four different cell organelles: c mitochondria (Mito-EGFP); d ER (Sec61B-EGFP); e peroxisome (PEX16-EGFP); and f nucleus (PTBP1-EGFP). Co-localisation images (right) of Ir-OA (red signal) and each EGFP (green signal). λex = 405 nm and 488 nm (Ir-OA and EGFP, respectively). Pearson’s coefficient for respective cell organelles with Ir-OA was calculated using Image J software (Mito-EGFP, R = 0.907; Sec61b-EGFP, R = 0.477; PEX15-EGFP, R = 0.124; PTBP1-EGFP, R = −0.347 vs. Ir-OA). Line-cut analysis (bottom) of western blot signals with or without photo-irradiation was performed to quantify the crosslinking efficiency (η) (n = 3). Each correlation value (R2) indicating similarity was written above the line cut spectrum. Note that higher efficiency for ER than mitochondria is for significant OMM/IMM location of Ir-OA. All imaging and blot experiments were repeated three times independently, and each experiment showed similar results. Source data are provided as a Source Data file.
Line-cut analysis comparing the presence or absence of light provided a correlation value (R2), which was utilised to calculate crosslinking efficiency (η) (calculation details are explained in Supplementary Information) (Fig. 4c–f). Photocrosslinking was significantly generated in the ER membrane (η = 60.3%), mitochondrial matrix (η = 33.2%), and peroxisome (η = 20.6%) but not in the nucleus (η = 1.0%). The crosslinking efficiency difference is affected by the possibility of contact between Ir-OA and EGFP of each organelle. Therefore, we transfected four different EGFP constructs again and imaged the EGFP with Ir-OA to investigate their proximity (Fig. 4c–f, right). The Pearson’s coefficients R of each image were then calculated (Mito-EGFP, R = 0.907; Sec61b-EGFP, R = 0.477; PEX15-EGFP, R = 0.124; PTBP1-EGFP, R = −0.347 vs. Ir-OA). Considering that the EGFP closer to Ir-OA is expected to be more easily crosslinked, it can be reasoned that the EGFP in the mitochondrial matrix, ER membrane, and peroxisome were more crosslinked than the EGFP in the nucleus. Notably, the crosslinking efficiency of ER membrane proteins was more significant than that of mitochondrial matrix proteins and peroxisome proteins because Ir-OA is located on the outer surface of the OMM and IMM. The ER membrane proteins were in direct contact with Ir-OA, while the contact between the proteins in the mitochondrial matrix and Ir-OA is limited by the IMM. Therefore, the Ir-OA molecules triggered relatively more protein crosslinking in the ER membrane than that in the mitochondrial matrix. In addition, the EGFP crosslinking efficiency was measured in HeLa cells; the tendency was similar to that of HEK293T cells (Supplementary Fig. 19). Protein crosslinking could be a possible way to increase viscosity in cells by inducing aggregation of mitochondrial or mitochondria-contacting organelle proteins (i.e., proteins in the mitochondrial matrix, ER membrane, and peroxisome). The protein crosslinking-induced viscous mitochondrial environment can affect diffusion-mediated cellular processes, such as mitochondrial metabolism, transport, and signalling—by reducing biomolecular diffusion and reaction rates. Consequently, the locally increased viscosity of mitochondria accelerates cell death.
Mitochondrial depolarisation and related oxidised-proteome
Mitochondrial oxidative stress triggers mitochondrial depolarisation, which is the collapse of MMP. To monitor the change of mitochondrial depolarisation, intramolecular energy transfer was utilised because its efficiency is highly dependent on the surrounding polarity, thereby providing a ratiometric emission property (Fig. 5a). In pure MeOH solvent, the excited Ir-OA (λex = 400 nm, absorption maximum of donor) exhibited poor emission of acceptor (λem = 555 nm) with strong emission of energy donor (λem = 450 nm) due to its inefficient intramolecular energy transfer under relatively hydrophobic conditions (Fig. 5b). However, increasing water content caused the emission of acceptor (λem = 555 nm) to become gradually enhanced while the donor emission (λem = 450 nm) was reduced as efficient energy transfer occurred with increasing polarity. This is because the increasing hydrophilic environment forced the energy donor (acedan derivative) to move closer to the iridium ligands through π–π interactions, resulting in enhanced energy transfer efficiency (See Supplementary Information and Supplementary Fig. 20). We also confirmed the emission intensity profile inside cells according to polarity changes through lambda scanning (Supplementary Fig. 21).
a Schematic illustrating polarity dependent energy transfer efficiency changes and (b) following ratiometric emission property changes depending on the H2O:MeOH ratio. c Ratiometric CLSM imaging of HeLa cells with Ir-OA according to giving oxidative stress. Ratiometric emission was observed 0, 10, 45, and 90 min after oxidative stress exposure (LED array, λ = 400 nm; light dose = 0.17 J cm−2) (top, scale bars = 50 μm), and the mitochondrial polarity change was monitored by the real-time ratiometric imaging during photoactivation within 60 s (bottom, scale bars = 20 μm). The CLSM instrument’s laser excited Ir-OA for real-time imaging (bottom). Ratio = (emission of acceptor, λem = 573–620 nm/emission of donor, λem = 420–480 nm). Normal mitochondria with high MMP: red (top) and green (bottom). All imaging was repeated three times independently, and each experiment showed similar results. Source data are provided as a Source Data file.
By utilising this property, we represented ratiometric images monitoring mitochondrial depolarisation by oxidative stress (ratio = emission of acceptor, λem = 573–620 nm/emission of donor, λem = 420–480 nm) using CLSM (Fig. 5c). The mitochondria of HeLa cells with Ir-OA without inducing oxidative stress maintained high mitochondrial polarity (red, high MMP). Conversely, most mitochondria became gradually depolarised (blue, low MMP) within 90 min after photoactivation (0.17 J cm−2) (Fig. 5c top). Furthermore, under continuous photoactivation, the emission ratio of Ir-OA exhibited an rapid change (green to blue) within 30 s in real-time analysis, implying rapid mitochondrial depolarisation by excessive oxidative stress (Fig. 5c bottom and Supplementary Movie 1). To support this, the conventional dye for detecting MMP loss, tetramethylrhodamine ethyl ester (TMRE), was utilised to confirm the time range of mitochondrial depolarisation. In HeLa cells with Ir-OA, the TMRE signal was completely quenched in 30 min after photoactivation (Supplementary Fig. 22). Consequently, we expect that proteins oxidised by a large amount of ROS are a primary cause of the strong and rapid depolarisation.
To identify proteins that are significantly affected by depolarisation, the oxidised proteome in the whole cell was profiled because severe protein oxidation is one of the critical induction points for protein dysfunction36,37. As methionine is a common and easily oxidised amino acid, we analysed the oxidised-methionine (O-Met) in the whole cell proteome (Supplementary Data). First, proteomes with substantial O-Met were sorted by label-free quantification (p-value < 0.1) (Fig. 6a). Protein oxidation by photoactivated Ir-OA mainly occurred in the mitochondria, ER, and vesicles, and proteins in the cytoplasm and nucleus were partially oxidised as well, which closely corresponded to the crosslinking efficiency results (Fig. 6b). Next, we investigated 28 mitochondrial proteins among 112 substantially O-Met mitochondrial proteins. These were categorised according to their function: (i) channel and translocase (ii) oxidative phosphorylation (OXPHOS) complex. The proteins were visualised by heat map imaging (Fig. 6c). Among these, we focused on the oxidation of the VDAC1, VDAC3, and SLC25 family for the channel and translocase group, and ATP5A1 and ATP5C1 for the OXPHOS complex group. The channel and translocase group is directly involved in MMP alteration because of mitochondrial cation exchange38,39, especially that of H+ and Ca2+. VDAC1 and VDAC3 are indispensable OMM proteins that all ions and metabolites must cross before arriving at the mitochondrial inner space. Ions and metabolites that enter the inner mitochondrial membrane are subsequently transported to the matrix by the SLC25 family constituting mitochondrial carriers (MCs)39,40. Among the SLC25 family members, five proteins (SLC25A3, SLC25A5, SLC25A6, SLC25A10, and SLC25A24) were also significantly oxidised. Collectively, Ir-OA photoactivation substantially collapsed the MMP owing to severe oxidation of the two representative gatekeepers on the outer and inner mitochondrial membranes, thereby disturbing the movement of ions or transport metabolites.
a Quantitatively analysed Volcano plot of oxidised proteome. The substantially oxidised proteome was sorted in the range of log2(Fold change) > 0 and –logP value > 1 (light blue region). b Proportion of the oxidised proteome in various organelles. Proteome location was determined using UniProt and mitochondria proteins were cross-checked with Human MitoCarta2.0 dataset consisting of 1158 human genes. The proportion (%) was obtained as ratio between the number of significantly oxidised proteome (−logP > 1) and that of whole oxidised proteome (−logP > 0) in each cell organelle. c Heat map with label free quantification (LFQ) values for 28 mitochondrial proteins among 112 substantially oxidised mitochondrial proteins (Supplementary Data). d Three representative oxidised proteins; OXPHOS complex I (NDUFS1 and NDUFA9), OXPHOS complex III, and voltage-dependent anion-selective channel 1 (VDAC1). The crystal structures of three proteins from RCSB protein data bank (PBD ID) were visualised and processed with PyMOL.
Our O-Met proteome also included components of the OXPHOS complex essential for the mitochondrial metabolic process (ATP5A, ATP5C1, CYC1, UQCRC1, NDUFS1, and NDUFA9). The OXPHOS complex is another target responsible for possible mitochondrial polarity changes following photoactivation of Ir-OA. The OXPHOS complex is involved in the vast majority of ATP production and H+ efflux/influx between the IMS and the matrix41,42. ATP5A1 and ATP5C1 are components of OXPHOS complex V and responsible for H+ influx, which is solely driven by OXPHOS complex V. Therefore, along with Ca2+ imbalance, H+ gradient collapse also contributes to mitochondrial depolarisation. Note that the crystal structures of the voltage-dependent anion channel (VDAC1), OXPHOS complex I matrix arm (NDUFS1 and NDUFA9), and OXPHOS complex III (CYC1 and UQCRC1) were described to indicate the O-Met site (Fig. 6d)43,44. Moreover, the above-mentioned VDAC1, VDAC3, and OXPHOS complex are closely associated with apoptosis39,45. This oxidative stress for each crucial protein could be related to the acceleration of oxidation-induced cell death. Thereby, we conclude that oxidation by Ir-OA photoactivation significantly affects not only mitochondrial depolarisation but also other mitochondrial functions and physiology.
Monitoring mitochondrial morphology change
The mitochondrial morphological changes and noticeable swelling are decisive evidence representing cell death progression46. We recorded the mitochondrial morphology in real-time by utilising time-lapse imaging during light irradiation (14 mW, 405 nm laser of laser scanning microscopy) (Fig. 7a, b). Interestingly, the shape of most mitochondria became round within 180 s, and the mitochondrial matrix swelling occurred with frequent fission and fusion (Fig. 7a, b and Supplementary Movies 2–4). In our O-Met proteome, several protease and chaperones were oxidised (Fig. 7c), which could damage their functions of eliminating and restoring un-/misfolded mitochondrial proteins. Therefore, it accumulated damaged proteins and triggered corresponding mitochondrial fission/fusion and swelling. Fission/fusion, known as the protein quality control process, could be related to oxidation of mitochondria/ER proteases and chaperones. Thus, fission/fusion is overloaded due to the accumulation of damaged proteins inside the mitochondria caused by the dysfunction of proteolysis. The phenomenon corresponds to the fact that the proteins involved in fission and fusion were not observed in the O-Met proteome (Fig. 7d). Further, mitochondrial matrix swelling could be explained by the oxidation of OXPHOS and channel proteins leading to an ion imbalance (Fig. 7e). The dysfunction of OXPHOS complex I must increase the NADH level and cause VDAC closure, increasing matrix Ca2+ concentration47,48; additionally, the Ca2+ level becomes elevated by the oxidation of LETM146, involved in Ca2+ efflux. The accumulation of Ca2+ inside the matrix, mitochondrial depolarisation, and ATP depletion caused by dysfunction of OXPHOS may trigger the mitochondrial permeability transition pore (MPTP) opening that accelerates influx of ions, water, and other solutes46,49,50. Accordingly, the MPTP opening swells the mitochondrial matrix, leading to cell death by abolishing the OMM to release cell death-inducing factors (Fig. 7e)46.
a Time-lapse Airyscan 2 images of HeLa cells with Ir-OA before light irradiation (14 mW, 405 nm laser of laser scanning microscopy) (left) and after irradiation for 204 s (right). To monitor morphological changes, the mitochondrial matrix was transfected by Mito-EGFP (green signal) (scale bars = 10 μm). White boxes indicate mitochondrial fission and fusion. The experiment was repeated three times independently, and each experiment showed similar results. b Enlarged time lapse images (0–340 s) of white boxes from Fig. 7a. Along with fission and fusion, mitochondria swelling was also observed. c Investigation of proteases and mitochondrial fission and fusion-related protein oxidation. Heat map diagram for O-Met proteins related to mitochondrial protein quality control, in addition to (d) fission, and fusion. e Brief description of a mechanism for mitochondrial environment change. This illustrates the impact of mitochondrial oxidative stress based on proteomic analysis of mitochondrial oxidative stress. f Mechanistic description of mitochondrial oxidation-induced cell death based on phenomenological observations and proteome analyses.








 User Center
User Center My Training Class
My Training Class Feedback
Feedback

















Comments
Something to say?
Log in or Sign up for free